How Many Electrons Are Shared in a Triple Bond
They can be identified by using a Lewis structureElectron pairs are therefore considered lone pairs if two electrons are paired but are. Bond Polarity of O-H bond in an H 2 O.
Question Video Recalling The Number Of Electrons In A Triple Covalent Bond Nagwa
In this one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms.

. Some molecules in which three pairs of electrons are shared by two atoms contain triple bonds. Two H atoms covalent bond- electrons shared 1 electron each. 4 outer electrons to form ethyne with triple bond.
The Spatial Arrangement of Various Bonds in the Atom or Molecule- The shared pair of electrons also experience dragging force from the other bonded and non-bonded pair of electrons. Shared pair electrons around carbon 4 single bonds 8. The neutral chemical species thus formed is called free radical.
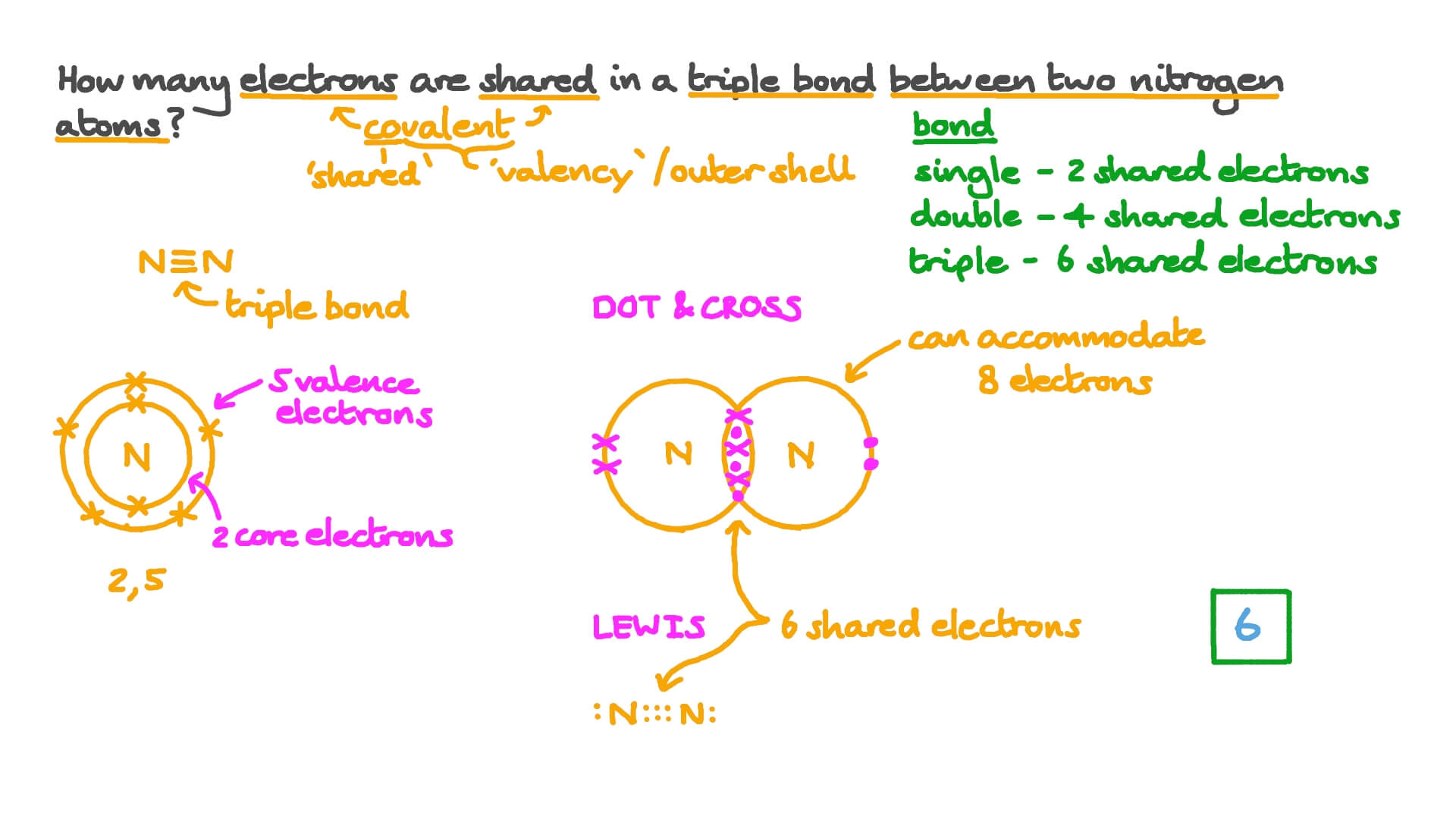
A simple compound that has a triple bond is nitrogen. Then compare the model to real molecules. The pair of electrons which are shared by the two atoms now extend around the nuclei of atoms leading to the creation of a molecule.
C As can be seen above pi electrons can move towards one of the two atoms they share to form a new lone pair. D pi electrons can also move to an adjacent position to make new pi bond. H H C C.
H H Methoxymethane dimethylether Combine two C atoms 4 electrons one O atom 6 electrons and six H atoms 1 electron. A typical double bond consists of one sigma bond and one pi bond. Lone pair electrons on carbon 0.
Stable molecule results at completed shell octet eight dots for main-group. Nitrogen belongs to group rmVleft rmA right of the periodic table and has 5 electrons in its outermost shell. Get help with your Chemical bond homework.
Ethyne C2H2 C. Lewis structures electron dot show valence electrons of an atom as dots Hydrogen has one dot representing its 1s electron Carbon has four dots 2s2 2p2 due to 4 e- in valence shell Kekulé structures line-bond structures have a line drawn between two atoms indicating a 2 e- covalent bond. Explore molecule shapes by building molecules in 3D.
D Number of bonds. Wills Molecular Stability - covalent vs ionic bonding. Generally homolytic fission takes place in non-polar covalent molecules in the presence of sunlight or high temperature.
Access the answers to hundreds of Chemical bond questions that are explained in a way thats easy for you to. Fission of a Covalent Bond. 4 0 82 o formal charge on the left side carbon atom.
Two pi bonds are the maximum that can exist between a given pair of. How does molecule shape change with different numbers of bonds and electron pairs. The value of bond order also predicts the number of bonds in the molecule.
Carbon monoxide CO has a total of 10 valence electrons. Such an unequal sharing of the pair of bonding electrons results in a POLAR bond. For example the sigma molecular orbital that serves to bond two fluorine atoms together is generated by the overlap of p-orbitals part A below and two sp 3 hybrid orbitals of carbon may combine to give a similar sigma orbital.
Ch01 Introduction landscapedocx Page 13 Bond Polarity A covalent bond where the electrons are shared equally is called a non-polar bond. In chemistry a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pairLone pairs are found in the outermost electron shell of atoms. In a single bond 2 electrons are shared in a double bond four electrons are shared and in a triple bond six electrons are shared.
Thus the triple bond is difficult to break since it is the strongest bond. Bond orders 1 2 3 signifies the presence of single double triple bonds respectively in a particular molecule. In covalent bond a greater number of electrons are shared among atoms then the stronger the bond exists.
When these bonding orbitals are occupied by a pair of electrons a covalent bond the sigma bond results. H-H Bonds between different atoms usually result in the electrons being attracted to one atom more strongly than the other. This results in different bond polarity among the same participating atoms that are present in other molecules.
A sigma bond latexsigmalatex forms when two atomic orbitals overlap between the nuclei of two atoms also known as the internuclear axis. Valence electrons of carbon 4. Valence bond and molecular orbital theories are used to explain chemical bonding.
Two atoms that have unpaired electrons in their orbitals can overlap to give rise to a chemical bond. Find out by adding single double or triple bonds and lone pairs to the central atom. To satisfy the octet rule for the carbon the two atoms form a triple bond with six shared electrons in three bonding molecular orbitals.
In the example above the pi electrons from the CO bond moved towards the oxygen to form a new lone pair. For the right side carbon atom. Shared pair electrons around carbon one triple bond and one single bond 8.
A covalent bond indicates the sharing of electrons between atoms. Compounds that contain carbon also called organic compounds commonly exhibit this type of chemical bonding. For example the CC double bond in ethylene H 2 CCH 2A typical triple bond for example in acetylene HCCH consists of one sigma bond and two pi bonds in two mutually perpendicular planes containing the bond axis.
Since four of the shared electrons come from the oxygen atom and only two from carbon one of the bonding orbitals is occupied by two electrons from.

Covalent Bonds Covalent Bonding Ionic Bonding Electron Configuration

2 7 Single Double And Triple Covalent Bonds Covalent Bonding Teaching Chemistry Chemistry Classroom



Comments
Post a Comment